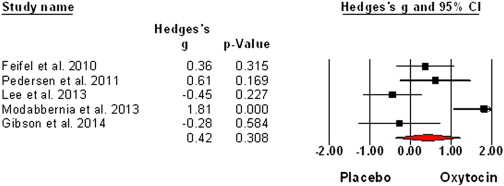
A single dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. (2015)
Schizophrenia is associated with significant impairments in both higher and lower order social cognitive performance and these impairments contribute to poor social functioning. People with schizophrenia report poor social functioning to be one of their greatest unmet treatment needs. Recent studies have suggested the potential of oxytocin as such a treatment, but mixed results render it uncertain what aspects of social cognition are improved by oxytocin and, subsequently, how oxytocin might best be applied as a therapeutic. The aim of this study was to determine whether a single dose of oxytocin improved higher-order and lower-order social cognition performance for patients with schizophrenia across a well-established battery of social cognition tests. Twenty-one male patients received both a single dose of oxytocin nasal spray (24IU) and a placebo, two weeks apart in a randomized within-subjects placebo controlled design. Following each administration, participants completed the social cognition tasks, as well as a test of general neurocognition. Results revealed that oxytocin particularly enhanced performance on higher order social cognition tasks, with no effects on general neurocognition. Results for individual tasks showed most improvement on tests measuring appreciation of indirect hints and recognition of social faux pas. These results suggest that oxytocin, if combined to enhance social cognition learning, may be beneficial when targeted at higher order social cognition domains. This study also suggests that these higher order tasks, which assess social cognitive processing in a social communication context, may provide useful markers of response to oxytocin in schizophrenia
“…it would be useful to identify reliable markers of response to oxytocin in different clinical populations, to be able to predict who is receiving adequate dosing and likely to respond to treatment. Currently, there is little understanding as to what constitutes a reliable response to oxytocin, although some social cognitive tests, such as emotion recognition, have shown promise as potential markers in healthy populations.
Thus, the aim of this study was to further explore the different domains of social cognition in patients with schizophrenia following intranasal administration of oxytocin. We utilized well-known and established tests of higher order and lower order social cognition to further test recent claims that oxytocin has specific effects on higher order in comparison to lower order social cognition tasks.”
“Participants received either 24 international units (IU) of oxytocin or placebo (4 IU per spray, 3 sprays per nostril) at each administration (Phases A and B). Nasal sprays were labeled with sequential numbers and Phase A or Phase B; blocking was in sets of 6 (3 active and 3 placebo sprays) in a randomly generated order. All research staff members conducting assessments and participants were blind to treatment allocation and unaware of randomization.”
“The results of this study showed that when conducting overall analysis, a single dose of oxytocin nasal spray improves performance overall on higher order social cognition. This effect was significant on two individual higher order social cognition tests, the hinting task and the non-faux condition of the Faux Pas Recognition Task. In contrast, we found improvement on only one lower order social cognition test, accuracy for detecting vocal intonations of affect, and no effect on general neurocognition. There was no effect on general neurocognition. Oxytocin was well tolerated and patients seemed unable to detect the active dose in this cross-over design.”
“Our finding that oxytocin improves higher order social cognition is consistent with previous research that suggests oxytocin effects may be particularly powerful for these higher order processes in schizophrenia that require more complex social cognitive processing. It is of note that the individual tasks that showed most benefit of oxytocin, the Faux Pas Recognition Task and the Hinting Task, involve appreciation of the social nuances in communicative exchanges. Further research is now needed to link changes in brain function during higher order social cognition tasks of this type to further understand the neurobiological basis of the impact of oxytocin on social cognition in schizophrenia. This research also suggests that oxytocin may have particular potential to provide adjunctive therapeutic benefit for patients when combined specifically with higher-order social cognition training treatment approaches.”
“As information about symptomatology was only assessed at the initial assessment, in accord with common practice using the SAPS and SANS to assess symptom severity over a month, we were unable to assess whether oxytocin had any significant impact on symptoms at the time of drug administration. Although oxytocin has been shown to exhibit therapeutic effects on positive and negative symptoms, this was observed after a two-week treatment with oxytocin (Pedersen et al., 2011), and psychotic symptoms are unlikely to shift with a single dose. We have also previously reported on the current limitations of oxytocin nasal spray administration (Guastella et al., 2013) in single dose and longitudinal studies. Future research should endeavor to improve current delivery methods of oxytocin to central and peripheral sites of action (Quintana et al., 2015). We did not assess a range of individual difference factors that have been proposed to moderate response to oxytocin (Bartz et al., 2011). These include polydipsia in schizophrenia (Goldman et al., 2011). We also acknowledge that our findings can only be generalized to a male schizophrenia population, and that the effects of oxytocin on other social cognitive abilities (e.g. social perception, attributional style etc.) have yet to be explored. Finally, we note the small sample size and the appearance of trends on other measures of social cognition could suggest that with more power oxytocin may influence other measures of social cognition (e.g., Reading the Mind in the Eyes Test).
In conclusion, the overall results of this study confirm the use of oxytocin nasal spray to enhance higher order social cognition performance in schizophrenia. Future studies are now needed to link these performance changes to neurobiological mechanisms (e.g., imaging, Aoki et al., 2015; physiological, Quintana et al., 2013) markers, and to link these changes to more functional measures of social behavior. Results of this study would also support further evaluation of the impact of combining oxytocin with social cognition training procedures (e.g., Cacciotti-Saija et al., 2015) but with a greater focus on learning programs that might enhance specifically higher-order social cognitive processes.”
Another study is also interesting:
Oxytocin promotes prosocial behavior, especially in those individuals who are low in affiliation (e.g., avoidantly attached individuals), but can exacerbate interpersonal insecurities in those preoccupied with closeness (e.g., anxiously attached individuals). One explanation for these opposing observations is that oxytocin induces a communal, other-orientation. Becoming more other oriented should help those people who focus on the self to the exclusion of others, but could be detrimental to those who are other focused but have little sense of an agentic self. Using a within-subjects design, we administered intranasal oxytocin and placebo to 40 males and measured their agency (self-orientation) and communion (other-orientation). Oxytocin produced a slight increase in communion for the average participant; however, as predicted, avoidantly attached individuals were especially likely to perceive themselves as more communal (“kind,” “warm,” “gentle,” etc.) after receiving oxytocin than after receiving the placebo. There was no main effect of oxytocin on agency for the average participant; however, anxiously attached individuals showed a selective decrease in agency (“independent,” “self-confident,” etc.) following administration of oxytocin. These data help explain the complex social effects of oxytocin.
I’ve tried both buccal (200IU) and intranasal oxytocin at various doses (exceeding 24IU) and for periods of up to a couple of weeks in the past without noticing anything subjectively. I was more focused on improving negative affect, most particularly relating to loneliness and didn’t specifically try to evaluate improvements in social cognition. My social skills are pretty crappy and I have an autism spectrum condition which adds to my social impairments, so this is an area of research I hope to see evolving as fast as possible.
See also:
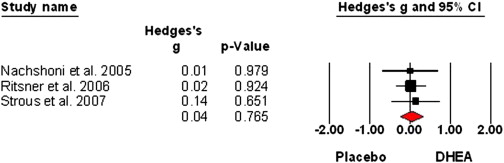
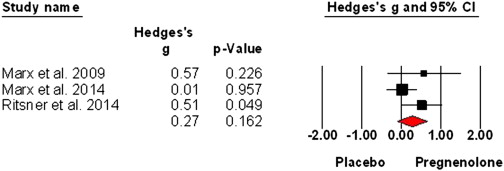
Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review (2015)