After several years of heavy smoking, I’ve now been without a cigarette for over a month. It’s been a hard journey to simply stop cigarettes, despite effectively switching to nicotine replacement therapies (patch and gum) and supplying my brain with the nicotine it craves. Interestingly, the nicotine replacements do not have the same therapeutic effectiveness towards improving attention, ablating negative affect and in reducing the intensity of auditory hallucinations. Within days of stopping smoking, I felt very depressed, impulsive and almost aggressive. Even a hit of nicotine via an e-cigarette wasn’t as rewarding as it used to be with tobacco. These feelings, particularly the aggression (which is not like me at all) have eased but I still feel ‘not quite right’.
It’s interesting to note the MAO-A and -B reductions in heavy smokers. PET scans using radiolabeled MAO-A and MAO-B inhibitor binding show some striking reductions – smokers have a much lower activity of peripheral and brain MAO-A (30%+) and -B (40%). [1] A different study found reductions of MAO-A in the order of 60% in cortical areas and 40% in the caudate and thalamus.

While some of the changes in my mental state could be explained by the effect of increased clozapine levels due to CYP1A2 activity normalising after stopping smoking, I suspect that changes in monoamine oxidase inhibition are the main cause.
One review states:
“Several early clinical studies analyzing platelet MAO-B and peripheral markers of MAO-A reported evidence that smokers had reduced MAO-B and MAO-A. At the time, it was not known if this was due to MAO inhibition by substances in tobacco smoke or to low rates of MAO synthesis in smokers or whether low MAO individuals were more vulnerable to smoking. Because these reports were based on peripheral measures of MAO-A activity, it was also not known if brain MAO was also lower in smokers. PET studies with [11C]clorgyline and [11C] l-deprenyl-D2 were the first to document low brain MAO-A and MAO-B in smokers relative to nonsmokers and former smokers with average reductions of brain MAO-A and MAO-B of 28% and 40%, respectively. Later PET studies confirmed early reports that nicotine does not inhibit brain MAO-B, that smoking a single cigarette does not produce a measurable change in brain MAO-B in nonsmokers, and that an overnight cigarette abstinence for smokers does not produce a measurable recovery of brain MAO-B activity. Widespread inhibition of cortical MAO-A inhibition in smokers was recently replicated with [11C]befloxatone and PET, and this study reported an even greater average inhibition of MAO-A (~60%) in a group of seven smokers and six healthy volunteers.
Following these studies, the hypothesis was tested that brain MAO-A levels would increase during acute withdrawal from cigarettes and that this would be related to plasma clearance of the potent MAO-A inhibitor, harman, an alkaloid that is present in tobacco smoke.Brain MAO-A VT as measured with [11C]harmine increased between active smoking and acute withdrawal and the percent change correlated with plasma levels of harman. As plasma harman levels dropped during the withdrawal period, MAO-A levels increased along with depression symptoms supporting the suggestion that the elevation of MAO-A during the acute withdrawal phase may account for depressed mood during the withdrawal from cigarettes. Contrasting with results with [11C]clorgyline and [11C]befloxatone, this study with [11C]harmine showed no significant differences in baseline brain MAO-A levels. Possible variables could include smoking dose or time between last cigarette and PET scan.”
Studies have found that inhibition of MAO activity by compounds present in tobacco smoke [2, 3] may combine with nicotine to produce the intense reinforcing properties of cigarette smoking that lead to addiction [4]. Antisocial behaviour [5], impulsiveness and aggression [6] have been linked to genetic and epigenetic regulation of MAO-A activity. Smoking has been found to induce long-lasting effects through epigenetic regulation of monoamine oxidases [7]
Interestingly, sensory gating is also modulated by both MAO-A activity and nicotine:
The separate and combined effects of monoamine oxidase inhibition and nicotine on P50 sensory gating
The cognitive effects of nicotine in humans remain a topic of great interest, due to the continued prevalence of cigarette smoking in society as well as the hypothesis that cognitively impaired populations such as schizophrenia patients use nicotine as a means of self-medicating against deficits of sensory gating. However, chronic smoking can predispose individuals to robust monoamine oxidase (MAO) inhibition, and thus far, the effect of MAO inhibition on human sensory gating is unknown. In this study, we investigated the effects of both nicotine (6-mg gum) and pharmacologically induced MAO-A inhibition via moclobemide (75 mg) on P50 event-related potential-indexed sensory gating in a sample of 24 healthy non-smoking males. Ratio score (rP50) measured gating revealed significant improvement in auditory stimulus suppression after combined nicotine and MAO-A inhibition compared to placebo and to the nicotine-alone condition. This nicotine + MAO-A inhibition-induced efficient gating was consistent regardless of participants’ baseline (placebo) gating efficiency, despite the observation that nicotine in the absence of MAO-A inhibition exhibited a detrimental effect on gating in participants with high baseline suppression ratios. Nicotine and monoamine oxidase-inhibiting agents in tobacco smoke appear to exert a synergistic effect on sensory gating, which may contribute to the elevated dependence rates seen in populations with cognitive deficits such as schizophrenia.
Studies suggest that pharmacological reduction of MAO-B levels during the early part of a quit attempt may aid in smoking cessation [8] and likewise, MAO-A inhibition is also promising [9] Studies in rodents have confirmed that a combination of MAO-A and MAO-B inhibition is also a possible option and potentially more effective than targeting a single isoform [10] Different tobacco types have varying inhibitory action towards MAOs [11]
Several studies have found selegiline is an effective adjunct to antipsychotics in reducing negative symptoms [12] and the antidepressant activity of MAO-A or non-selective inhibitors is well established.
Interestingly, there are sex differences in cigarette reward pathways in men and women and these may be influenced by differences in monoamine oxidase activity [13]
While I’m on medications that are contraindicated by concomitant MAOI use, an interesting therapeutic strategy could be partial inhibition of MAO in suitable candidates. With substantial MAO inhibition, particularly with an irreversible inhibitor, there are serious risks such as the induction of serotonin syndrome when combined with serotonergic agents or potentiation of pressor amine responses but nonetheless, the cautious combination of a MAO inhibitor plus a TCA/SSRI has been shown to be of value in treatment-resistant depression (TRD) [14]. Such strategies are risky, the authors concluding that they should be “employed with extreme caution.”
It would be interesting if low doses of a reversible MAOI such as moclobemide, low doses of selegiline, or perhaps a combination of both – alongside nicotine replacement therapy – could be implemented to produce the same therapeutic benefits tobacco smoking provides for many people with schizophrenia, without the harm.
Moclobemide at clinical doses reversibly inhibits ~80% MAO-A activity for 8-10 hrs when administered acutely [15] while doses of 150mg t.i.d for 4 days decreased MAO-B activity by 32% [16] Lower, sub-therapeutic doses administered chronically may be able to provide MAO-A inhibition similar to that of smoking and the combination with low doses of selegiline, even administered weekly*, may likewise provide MAO-B inhibition to a similar extent. Due to the reversible nature of moclobemide, initial results suggest that combination with a SSRI does not produce a serotonin syndrome in most cases and that the combination is a potent treatment option for patients with TRD [17]
* Because the binding of selegiline to MAO-B involves covalent modification of the enzyme, the 40-day half-life for recovery of brain MAO-B activity is a measure of the half-life for the synthesis of new MAO-B molecules. Moreover, the slow turnover of brain MAO-B suggests that reduced dosing of selegiline should be evaluated and may have an impact on reducing the side effects and the costs arising from excessive drug use.
To conclude [18]:
“…recent animal studies have shown that a long-term treatment with MAO inhibitors enhances dramatically the rewarding and reinforcing effects of nicotine. This property seems to be related to MAO-A rather than MAO-B inhibition. Our current results suggest that also in humans, the cerebral MAO-A inhibition associated with smoking can potentiate the pharmacological effects of nicotine and thus participate in the establishment of tobacco addiction. Because MAO-A inhibitors are considered effective antidepressant drugs, our results raise the question of a possible mood-enhancing effect of smoking. To our knowledge, there are no published data in patients about the degree of cerebral MAO-A inhibition required for an antidepressive effect.
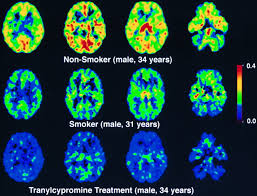
In healthy subjects, a mean 58% and a 64% to 79% inhibition of cerebral MAO-A have been reported with a low dose (10 mg/d) of the nonselective MAO inhibitor tranylcypromine and with 150 mg BID (a common therapeutic dose) of the selective MAO-A inhibitor moclobemide, respectively. In our current data, the degree of cortical MAO-A inhibition in smokers approached those earlier observations. It can thus be speculated that smoking-associated MAO-A inhibition might have a mood-modulating effect in smokers. Recently, Meyer et al found in untreated nonsmoking depressive patients an average 34% elevation of cerebral MAO-A density, supporting that a hyperactivity of MAO-A which reduces monoamine levels could be a primary process in depression. If this is true, smoking might, at least in some patients, counteract the elevation of MAO-A activity and thus prevent or ameliorate the depressive symptoms. This mechanism could also explain the high incidence of depressive relapses after smoking cessation in smokers with a previous history of major depression.”
Similarly, 5-HT2 receptor binding is impacted by nicotine administration [21]:
“…chronic nicotine administration in home cages induces bi-directional neuroplastic changes within 5-HT2A and 5-HT2C receptors in the prefrontal cortex. Pairing the nicotine with environmental context potentiates the neuroplastic response in the latter region and evokes opposite changes in 5-HT2A receptor binding in striatal and tegmental regions compared with nicotine administered in the absence of the context, indicating a modulatory role of environmental context..”
See also: