 Epigenetic memory: the Lamarckian brain.
Epigenetic memory: the Lamarckian brain.
“Recent data support the view that epigenetic processes play a role in memory consolidation and help to transmit acquired memories even across generations in a Lamarckian manner.“

Cognitive appraisals of stressors may lead to differing levels of prenatal maternal stress and have transgenerational effects:
“[A] pregnant women’s cognitive appraisals of an independent stressor may have widespread effects on DNA methylation across the entire genome of their unborn children, detectable during adolescence” [link]
The recent sociodevelopmental cognitive model of schizophrenia/psychosis is a highly influential and compelling compendium of research findings. Here, we present logical extensions to this model incorporating ideas drawn from epigenetic mediation of psychiatric disease, and the plausible effects of epigenetics on the emergence of brain network function and dysfunction in adolescence. We discuss how gene–environment interactions, effected by epigenetic mechanisms, might in particular mediate the stress response (itself heavily implicated in the emergence of schizophrenia). Next, we discuss the plausible relevance of this framework for adolescent genetic risk populations, a risk group characterized by vexing and difficult-to-explain heterogeneity. We then discuss how exploring relationships between epigenetics and brain network dysfunction (a strongly validated finding in risk populations) can enhance understanding of the relationship between stress, epigenetics, and functional neurobiology, and the relevance of this relationship for the eventual emergence of schizophrenia/psychosis. We suggest that these considerations can expand the impact of models such as the sociodevelopmental cognitive model, increasing their explanatory reach. Ultimately, integration of these lines of research may enhance efforts of early identification, intervention, and treatment in adolescents at-risk for schizophrenia.
Stressor exposure during early life has the potential to increase an individual’s susceptibility to a number of neuropsychiatric conditions such as mood and anxiety disorders and schizophrenia in adulthood. This occurs in part due to the dysfunctional stress axis that persists following early adversity impairing stress responsivity across life. The mechanisms underlying the prolonged nature of this vulnerability remain to be established. Alterations in the epigenetic signature of genes involved in stress responsivity may represent one of the neurobiological mechanisms. The overall aim of this review is to provide current evidence demonstrating changes in the epigenetic signature of candidate gene(s) in response to early environmental adversity. More specifically, this review analyses the epigenetic signatures of postnatal adversity such as childhood abuse or maltreatment and later-life psychopathology in human and animal models of early life stress. The results of this review shows that focus to date has been on genes involved in the regulation of hypothalamic-pituitary-adrenal (HPA) axis and its correlation to subsequent neurobiology, for example, the role of glucocorticoid receptor gene. However, epigenetic changes in other candidate genes such as brain-derived neurotrophic factor (BDNF) and serotonin transporter are also implicated in early life stress (ELS) and susceptibility to adult psychiatric disorders. DNA methylation is the predominantly studied epigenetic mark followed by histone modifications specifically acetylation and methylation. Further, these epigenetic changes are cell/tissue-specific in regulating expression of genes, providing potential biomarkers for understanding the trajectory of early stress-induced susceptibility to adult psychiatric disorders.
Environmental Stressors and Epigenetic Control of the Hypothalamic-Pituitary-Adrenal Axis
In this review, we provide a brief summary of several key studies that broaden our understanding of stress and its epigenetic control of the function and behavior of the hypothalamic-pituitary-adrenal (HPA) axis. Clinical and animal studies suggest a link among exposure to stress, dysregulation of the HPA axis, and susceptibility to neuropsychiatric illnesses. Recent studies have supported the notion that exposure to glucocorticoids and stress in various forms, durations, and intensities during different periods of development leads to long-lasting maladaptive HPA axis response in the brain. They demonstrate that this maladaptive response is comprised of persistent epigenetic changes in the function of HPA axis-associated genes that govern homeostatic levels of glucocorticoids. Stressors and/or disruption of glucocorticoid dynamics also target genes such as brain-derived neurotrophic factor (BDNF) and tyrosine hydroxylase (TH) that are important for neuronal function and behavior. While a definitive role for epigenetic mechanisms remains unclear, these emerging studies implicate glucocorticoid signaling and its ability to alter the epigenetic landscape as one of the key mechanisms that alter the function of the HPA axis and its associated cascades. We also suggest some of the requisite studies and techniques that are important, such as additional candidate gene approaches, genome-wide epigenomic screens, and innovative functional and behavioral studies, in order to further explore and define the relationship between epigenetics and HPA axis biology. Additional studies examining stress-induced epigenetic changes of HPA axis genes, aided by innovative techniques and methodologies, are needed to advance our understanding of this relationship and lead to better preventive, diagnostic, and corrective measures. 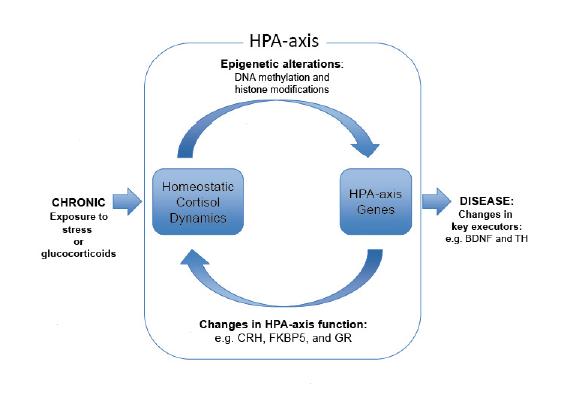 Stress-induced Perinatal and Transgenerational Epigenetic Programming of Brain Development and Mental Health
Stress-induced Perinatal and Transgenerational Epigenetic Programming of Brain Development and Mental Health
Research efforts during the past decades have provided intriguing evidence suggesting that stressful experiences during pregnancy exert long-term consequences on the future mental wellbeing of both the mother and her baby. Recent human epidemiological and animal studies indicate that stressful experiences in utero or during early life may increase the risk of neurological and psychiatric disorders, arguably via altered epigenetic regulation. Epigenetic mechanisms, such as miRNA expression, DNA methylation, and histone modifications are prone to changes in response to stressful experiences and hostile environmental factors. Altered epigenetic regulation may potentially influence fetal endocrine programming and brain development across several generations. Only recently, however, more attention has been paid to possible transgenerational effects of stress. In this review we discuss the evidence of transgenerational epigenetic inheritance of stress exposure in human studies and animal models. We highlight the complex interplay between prenatal stress exposure, associated changes in miRNA expression and DNA methylation in placenta and brain and possible links to greater risks of schizophrenia, attention deficit hyperactivity disorder, autism, anxiety – or depression-related disorders later in life. Based on existing evidence, we propose that prenatal stress, through the generation of epigenetic alterations, becomes one of the most powerful influences on mental health in later life. The consideration of ancestral and prenatal stress effects on lifetime health trajectories is critical for improving strategies that support healthy development and successful aging
DNA methylation and demethylation as targets for antipsychotic therapy
Schizophrenia (SZ) and bipolar disorder (BPD) patients show a downregulation of GAD67, reelin (RELN), brain-derived neurotrophic factor (BDNF), and other genes expressed in telencephalic GABAergic and glutamatergic neurons. This downregulation is associated with the enrichment of 5-methylcytosine and 5-hydroxymethylcytosine proximally at gene regulatory domains at the respective genes. A pharmacological strategy to reduce promoter hypermethylation and to induce a more permissive chromatin conformation is to administer drugs, such as the histone deacetylase (HDAC) inhibitor valproate (VPA), that facilitate chromatin remodeling. Studies in mouse models of SZ indicate that clozapine induces DNA demethylation at relevant promoters, and that this action is potentiated by VPA. By activating DNA demethylation, clozapine or its derivatives with VPA or other more potent and selective HDAC inhibitors may be a promising treatment strategy to correct the gene expression deficits detected in postmortem brain of SZ and BPD patients.
⇒ DNA methylation in psychosis: insights into etiology and treatment.
Implications of epigenetic modulation for novel treatment approaches in patients with schizophrenia
Schizophrenia is a heterogeneous and complex mental disorder with high rates of disability, nonrecovery, and relapse. The primary pharmacological treatments for schizophrenia are antipsychotics. Notwithstanding the efficacy of antipsychotics in ameliorating positive symptoms and reducing relapse rates, cognitive deficits and negative symptoms are not sufficiently treated with available pharmaceutical agents. Moreover, schizophrenia is associated with consistent, replicable, and clinically significant deficits in cognition. The importance of cognitive deficits in schizophrenia is emphasized by reports indicating that the severity of cognitive deficits is predictive of treatment compliance, adherence, and risk of relapse among first-episode individuals. Taken together, this review highlights epigenetic modulations involving histone deacetylase (HDAC) inhibitors as a potential avenue for novel treatment toward improvements in cognition and functional outcomes in patients with schizophrenia. The combination of epigenetic modulation with pharmacological interventions that engage multiple disparate physiological systems implicated in schizophrenia are discussed, and may represent a more effective strategy in ameliorating cognitive deficits and mitigating symptoms for improved functionality.
Epigenetic signaling in schizophrenia.
Histone modifications and DNA methylation represent central dynamic and reversible processes that regulate gene expression and contribute to cellular phenotypes. These epigenetic marks have been shown to play fundamental roles in a diverse set of signaling and behavioral outcomes. Psychiatric disorders such as schizophrenia and depression are complex and heterogeneous diseases with multiple and independent factors that may contribute to their pathophysiology, making challenging to find a link between specific elements and the underlying mechanisms responsible for the disorder and its treatment. Growing evidences suggest that epigenetic modifications in certain brain regions and neural circuits represent a key mechanism through which environmental factors interact with individual’s genetic constitution to affect risk of psychiatric conditions throughout life. This review focuses on recent advances that directly implicate epigenetic modifications in schizophrenia and antipsychotic drug action.
⇒ Methylated Spirits: Epigenetic Hypotheses of Psychiatric Disorders
Both messenger RNA and protein expression of HDACs 1, 3, and 5 have been shown to be altered in depression, bipolar disorder, and schizophrenia.
DA
“cortical neurons undergo a profound epigenetic reprogramming in response to dysfunctional D2 autoreceptor signaling leading to altered DA levels, a process that may underlie a number of neuropsychiatric disorders.” [1]
D1 receptor activation via clozapine has been found to ameliorate epigenetic and behavioral abnormalities induced by phencyclidine. [2]
Most of the antipsychotic drugs such as benzamides, clozapine, and lurasidone are epigenetic modifiers. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. [link]
HDACs are altered by atypical antipsychotic treatment:
“…expression of HDAC2, but not HDAC1 or HDAC4, is elevated in the frontal cortex of schizophrenic patients treated with atypical antipsychotics.”
GABA/Glu
“an important role of GABA was found in controlling the epigenetic and gene transcriptional responses to psychological stress. Thus, psychologically stressful events evoke a long-term impact on behavior through changes in hippocampal function brought about by distinct glutamatergic and glucocorticoid-driven changes in epigenetic regulation of gene transcription, which are modulated by (local) GABAergic interneurons and limbic afferent inputs. These epigenetic processes may play an important role in the etiology of stress-related mental disorders ” [3]

5-HT1A receptor-mediated histone acetylation has been implicated in epigenetic regulation of resistance to emotional stress. [4] Data suggests a key role for 5-HT1A receptor and BDNF-trkB signalling in driving a transitory epigenetic remodelling of chromatin structure that underlies neuronal plasticity [5]
Epigenetic dysregulation of the 5-HT2A receptor is implicated in schizophrenia. Chronic administration of atypical antipsychotics modulates the transcription of metabotropic glutamate 2 receptors via a serotonin 5-HT2A receptor–dependent upregulation and increased binding of HDAC2 to the mGlu2 promoter:
“Epigenetic dysregulation of HTR2A may contribute to SCZ, BD and earlier age of disease onset. Further research is required to delineate the dysregulation of other components of serotoninergic pathway to design new therapeutics based on the downstream effects of serotonin” [6]
“HDAC inhibitors prevented the repressive histone modifications induced at the mGlu2 promoter by atypical antipsychotics, and augmented their therapeutic-like effects. These observations support the view of HDAC2 as a promising new target for schizophrenia treatment.” [7]
Serotonin transporter methylation modifies the effect of the number of traumatic events on the risk of developing psychiatric disorders [8] and has been implicated in schizophrenia:
“[there is] evidence that the promoter region of the SERT gene is hypermethylated in patients with schizophrenia compared to control subjects. Intriguingly, this hypermethylation was most pronounced in antipsychotic-naïve patients. Conversely, methylation levels approached those of control subjects in patients treated with antipsychotics, with a correlation between duration of treatment and normalization of methylation levels.” [9]
HDAC-dependent epigenetic mechanisms are likely involved in the efficacy of SSRI antidepressant drugs:
“…reducing HDAC activity in fluoxetine-treated Balb/c mice leads to robust antidepressant and anxiolytic effects. While reducing the activity of class I HDACs 1 and 3 led to antidepressant effects, additional class II HDAC inhibition was necessary to exert anxiolytic effects. In fluoxetine-treated mice, HDAC inhibitors increased enrichment of acetylated histone H4 protein and RNA polymerase II at promotor 3 of the brain-derived neurotrophic factor (Bdnf) gene and increased Bdnf transcription from this promotor. Reducing Bdnf-stimulated tropomyosin kinase B receptor activation in fluoxetine-treated mice with low HDAC activity abolished the behavioral effects of fluoxetine, suggesting that the HDAC-triggered epigenetic stimulation of Bdnf expression is critical for therapeutic efficacy” [10]
Inhibition of HDACs increases 5-HT synthesis and release by epigenetic mechanisms and this increase in 5-HT release is mediated by the enhancement of AMPAR-mediated excitatory inputs and CaMKII signaling [link]
See more: Epigenetic Mechanisms of Serotonin Signaling
Other:
DNA methylation of oxytocin receptor (OXTR) decreases expression of the gene and high levels of methylation have been associated with autism spectrum disorders (ASD) and may play a role in other psychiatric disorders where social behaviour is impacted:
“…this link between epigenetic variability and social phenotype allows for the possibility that social processes are under epigenetic control.” [11]
“…high levels of OXTR methylation were associated with greater amounts of activity in regions associated with face and emotion processing including amygdala, fusiform, and insula. Importantly, we found that these higher levels of OXTR methylation were also associated with decreased functional coupling of amygdala with regions involved in affect appraisal and emotion regulation. These data indicate that the human endogenous oxytocin system is involved in attenuation of the fear response, corroborating research implicating intranasal oxytocin in the same processes. Our findings highlight the importance of including epigenetic mechanisms in the description of the endogenous oxytocin system and further support a central role for oxytocin in social cognition. This approach linking epigenetic variability with neural endophenotypes may broadly explain individual differences in phenotype including susceptibility or resilience to disease.”
⇒ Decreased methylation of OXTR has been reported in social anxiety disorder
Steroids play a role in the epigenetic regulation of social behaviours:
“…methylation patterns on some steroid-responsive genes were actively maintained by the presence of circulating steroid hormones that affect the expression of the socially relevant peptide vasopressin (AVP) and ERα within the bed nucleus of the stria terminalis (BNST).” [12]
Pharmacological activation of mGlu2/3 receptors has a strong impact on the epigenetic regulation of genes that have been linked to the pathophysiology of schizophrenia. [13]
HDAC2 induction and downregulation of NMDA receptor-related genes via histone deacetylation plays a role in the mechanism of electroconvulsive shock therapy [14]
Epigenetic regulation of BDNF likely plays a role in stress resilience:
“…data support the importance of hippocampal BDNF regulation in response to stressful events. Moreover, we identify a specific and adaptive regulation of bdnf exon VI in the hippocampus as a critical regulator of stress resilience, and strengthen the importance of epigenetic factors in mediating stress-induced adaptive and maladaptive responses in different individuals.” [15]
Histone acetylation may play a role in behavioural responses to social stress:
“…results suggest that changes in histone acetylation and expression of histone modifying enzymes in these regions correlate with the behavioral response to stress in socially defeated rats” [16]
Meditation may regulate HDACs:
“The regulation of HDACs and inflammatory pathways may represent some of the mechanisms underlying the therapeutic potential of mindfulness-based interventions. Our findings set the foundation for future studies to further assess meditation strategies for the treatment of chronic inflammatory conditions.” [17]
Exercise reverses behavioural deficits induced by social defeat stress:
“…exercise normalized social defeat-induced increase in oxidative stress, most likely by adjusting antioxidant response. Our data suggests involvement of epigenetic mechanisms including histone acetylation of H3 and modulation of methyl-CpG-binding in the hippocampus that might contribute to the rescue effects of exercise in social defeat-induced behavioral deficits in rats.” [18]
Dietary modifications: 
DNA methylation is one of the essential factors in the control of gene expression. Alteration of the DNA methylation pattern has been linked to various neurological, behavioral, and neurocognitive dysfunctions. Recent studies have pointed out the importance of epigenetics in brain development and functions including learning and memory. Nutrients related to one-carbon metabolism are known to play important roles in the maintenance of genomic DNA methylation. Previous studies have shown that the long-term administration of a diet lacking essential one-carbon nutrients such as methionine, choline and folic acid (methyl donors) caused global DNA hyper-methylation in the brain. Therefore, the long-term feeding of a methyl-donor-deficient diet may cause abnormal brain development including learning and memory. To confirm this hypothesis, 3-week-old mice were maintained on a folate-, methionine- and choline-deficient (FMCD) or control (CON) diet for 3 weeks. We found that the methyl-donor deficiency impaired both novel object recognition and fear extinction after 3 weeks of treatment. The FMCD group showed spontaneous recovery of fear that differed from that in CON. In addition, we found decreased Gria1 gene expression and specific CpG hyper-methylation of the Gria1 promoter region in the FMCD hippocampus. Our data suggest that a chronic dietary lack of methyl donors in the developmental period affects learning, memory and gene expressions in the hippocampus
Early exposure to dietary omega-3 fatty acids orchestrates key interactions between metabolic signals and Bdnf methylation creating a reservoir of neuroplasticity that can protect the brain:
“Results support a model in which diet can build an “epigenetic memory” during brain formation that confers resilience to metabolic perturbations occurring in adulthood.” [20]
Methionine is an epigenetically active amino acid that can exacerbate psychosis. HDAC inhibition reverses schizophrenia-like epigenetic behavioural modifications induced in animals by methionine administration [21]
Folate plays a role in psychiatric disorders:
“Interestingly, there is evidence that folate (folic acid) is lacking in depression and its deficiency may not only compromise active DNA formation but also methylation of both histones and DNA. Antidepressant-like actions of folate have been reported in rodents and studies are being pursued of its administration alone and together with SSRIs to patients” [22]
L-methylfolate has also been implicated in epigenetic regulation and has been used as a therapeutic intervention [23]
Melatonin causes epigenetic effects by modulating both DNA methylation and histone acetylation pathways. Recent reports have indicated that melatonin has an effect on histone modification. [24]
Melatonin induces histone hyperacetylation in vivo [a, b] Prenatal dexamethasone exposure induces gross alterations in hippocampal morphology and reduces hippocampal mRNA expression of reelin (reln) and GAD1. Melatonin has a beneficial effect in restoring hippocampal reln mRNA expression by reducing DNMT1 and MeCP2 binding to the reln promoter. [c]
Vitamin D may regulate epigenetic events. An active metabolite of vitamin D, 1,25-dihydroxyvitamin D(3) plays an essential role in cellular metabolism and differentiation via its nuclear receptor (VDR) that cooperates with several other chromatin modification enzymes (histone acetyltransferases and histone deacetylases), thereby mediating complex epigenetic events in vitamin D signaling and metabolism [25].
From Epigenetics – Psychosis – Schizophrenia – Bipolar Disorders
- The availability of methyl donors and cofactors is a major external influence on DNA methylation and the development of neuropsychiatric disorders.
- Yet an estimation of the impact deriving from an aberrant one-carbon metabolism is difficult and not straightforward
- It was postulated that altered one-carbon metabolism with an increase in brain SAM may be associated with DNMT1 overexpression and may become a crucial factor mediating:
- DNA hypermethylation and transcriptional down-regulation of putative candidate genes in cortical GABAergic neurons of SCZ or BP patients
- In contrast, a negative correlation between plasma Hcy and global DNA methylation has been reported
- SAM is effective for depression BUT [may] amplify the manic phase in bipolar disorders
- Try to reduce homocysteine levels with vitamin B6
- Omega-3 could be useful for the prevention and treatment of psychosis
- Synaptic plasticity
- Learning and memory processes
- It is difficult to estimate the impact of specific natural dietary sources on cellular epigenomic patterns, since dietary nutrients are normally ingested in complex combinations, and they interact with each other in their normal metabolic and physiological functions; consequently, identifying effective components is difficult
- Western diet is involved in the pathogenesis of psychosis
- Transgenerational effects can be a nightmare for future generations
 Epigenetic regulation of the immune system via diet has potential relevance:
Epigenetic regulation of the immune system via diet has potential relevance:
“There is increasing evidence that the epigenetic mechanisms that regulate gene expression during immune differentiation are directly affected by dietary factors or indirectly through modifications in gut microbiota induced by different dietary habits. Short-chain fatty acids, in particular butyrate, produced by selected bacteria stains within gut microbiota, are crucial players in this network.” [26]
Dietary polyphenols may also act via epigenetic mechanisms [27]
Epigenetic mechanisms regulated by polyphenols:Epigallocatechin-3-gallate: DNMT1 inhibition, HDAC inhibition, miRNAs repressionCurcumin: HAT inhibitionQuercetin: HAT activation and HDAC inhibitionGenistein: Histone demethylation, HAT activation and SIRT inhibition
See more:
Therapeutic perspectives of epigenetically active nutrients
The Influence of Early Life Nutrition on Epigenetic Regulatory Mechanisms of the Immune System
Other roles for HDAC inhibitors:
Recently, selective inhibitors of HDAC2 have been found to have cognitive enhancing properties, the authors concluding:
“selective pharmacological inhibition of HDAC2 is feasible and that inhibition of the catalytic activity of this enzyme may serve as a therapeutic approach towards enhancing the learning and memory processes that are affected in many neurological and psychiatric disorders” [28]
HDAC2 inhibitors also possess antidepressant properties in rodents. [29]
HDAC inhibitors may be effective cognitive enhancers in fear, anxiety and trauma therapy [30]
“HDAC inhibitors that enhance cued fear extinction may show translational promise for the treatment of fear-related disorders, including post-traumatic stress disorder (PTSD)” [review]
Stress resilience can possibly be enhanced pharmacologically:
“Brain-penetrant histone deacetylase 6 inhibitors increase Hsp90 acetylation and modulate GR chaperone dynamics offering a promising strategy to curtail deleterious socioaffective effects of stress and glucocorticoids.” [31]
Histone deacetylase inhibitors modulate other social behaviours:
“Recently, the involvement of histone modifications in the regulation of pair bonding has been established. Female prairie voles treated with histone deacetylase inhibitors (HDACi) became bonded to their mates in the absence of a mating event. This was accompanied by a specific upregulation of oxytocin receptor (oxtr) and vasopressin V1a receptor (avpr1a) in the nucleus accumbens (NAcc), which was associated with an increase in histone acetylation at their respective promoters, similar to that observed when untreated females were mated and formed a pair bond. However, HDACi treatment did not promote vasopressin and oxytocin receptor expression in the NAcc of female prairie voles that were not exposed to males, indicating that other factors related to social context are required to induce pair bonds.” [32]
Other medications acting via epigenetic mechanisms:
Lithium-induced neuroprotection is dependent on epigenetic modification of specific BDNF gene promoters and altered expression of apoptotic-regulatory proteins [33]
Long-term imipramine treatment increases N-methyl-D-aspartate receptor activity and expression via epigenetic mechanisms [34]
Epigenetic modifications are common with many medications:
“There is a significant increase of histone H3 acetylation in the nucleus accumbens after 15 days of treatment with sodium valproate, lithium chloride, lamotrigine, levetiracetam, olanzapine, clozapine, clomipramine, (S)-citalopram oxalate, or duloxetine hydrochloride. In the striatum, treatment with clomipramine, (S)-citalopram oxalate, duloxetine hydrochloride, mirtazapine, carbamazepine, lamotrigine, levetiracetam, olanzapine, or clozapine results in significant increases of both HDAC2 and HDAC3 expression. In the cingulate cortex, clomipramine, mirtazapine, sodium valproate, carbamazepine, lamotrigine, levetiracetam, olanzapine, or clozapine significantly increases HDAC3 expression. In the amygdala, the drugs (S)-citalopram oxalate, duloxetine hydrochloride, mirtazapine, sodium valproate, carbamazepine, lamotrigine, levetiracetam, and olanzapine all result in significant increases in HDAC5 expression. Interestingly, the antidepressant imipramine has been shown to selectively down-regulate HDAC5 in the hippocampus of a mouse model of depression. Moreover, overexpression of HDAC5 has been shown to block the antidepressive effects of imipramine in that model. Taken together, these data suggest that directly targeting HDACs may be an alternate way to attenuate depression and mood disorders.”
“Clozapine treatment is associated with decrease in histone H3 acetylation at the mGluR2 promoter and subsequent down-regulation of mGluR2 gene expression in both human and mouse frontal cortex” [35]
Neuroepigenetic regulation of pathogenic memories
Our unique collection of memories determines our individuality and shapes our future interactions with the world. Remarkable advances into the neurobiological basis of memory have identified key epigenetic mechanisms that support the stability of memory. Various forms of epigenetic regulation at the levels of DNA methylation, histone modification, and noncoding RNAs can modulate transcriptional and translational events required for memory processes. By changing the cellular profile in the brain’s emotional, reward, and memory circuits, these epigenetic modifications have also been linked to perseverant, pathogenic memories. In this review, we will delve into the relevance of epigenetic dysregulation to pathogenic memory mechanisms by focusing on 2 neuropsychiatric disorders perpetuated by aberrant memory associations: substance use disorder and post-traumatic stress disorder. As our understanding improves, neuroepigenetic mechanisms may someday be harnessed to develop novel therapeutic targets for the treatment of these chronic, relapsing disorders.