Expanding on ‘Schizophrenia and the Effects of Estrogen on Schizophrenia‘:
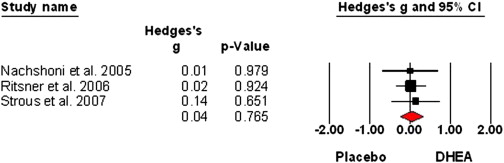
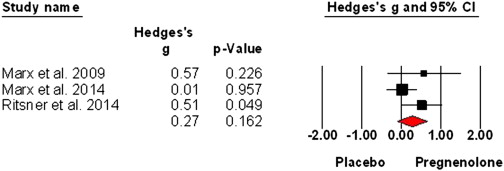
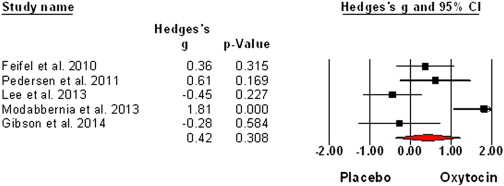
Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review (2015) concludes:
“…estrogen augmentation in women warrants further investigation, as beneficial effects on total, positive and negative symptom severity were found. However, risks of side effects of estrogens, partially associated with longer duration use, should be taken into account. For this reason, SERMs may serve as a favorable alternative for estrogens that is also appropriate for use in men.”
Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia.
There is increasing clinical and molecular evidence for the role of hormones and specifically estrogen and its receptor in schizophrenia. A selective estrogen receptor modulator, raloxifene, stimulates estrogen-like activity in brain and can improve cognition in older adults. The present study tested the extent to which adjunctive raloxifene treatment improved cognition and reduced symptoms in young to middle-age men and women with schizophrenia. Ninety-eight patients with a diagnosis of schizophrenia or schizoaffective disorder were recruited into a dual-site, thirteen-week, randomized, double-blind, placebo-controlled, crossover trial of adjunctive raloxifene treatment in addition to their usual antipsychotic medications. Symptom severity and cognition in the domains of working memory, attention/processing speed, language and verbal memory were assessed at baseline, 6 and 13 weeks. Analyses of the initial 6-week phase of the study using a parallel groups design (with 39 patients receiving placebo and 40 receiving raloxifene) revealed that participants receiving adjunctive raloxifene treatment showed significant improvement relative to placebo in memory and attention/processing speed. There was no reduction in symptom severity with treatment compared with placebo. There were significant carryover effects, suggesting some cognitive benefits are sustained even after raloxifene withdrawal. Analysis of the 13-week crossover data revealed significant improvement with raloxifene only in attention/processing speed. This is the first study to show that daily, oral adjunctive raloxifene treatment at 120 mg per day has beneficial effects on attention/processing speed and memory for both men and women with schizophrenia. Thus, raloxifene may be useful as an adjunctive treatment for cognitive deficits associated with schizophrenia.
‘The Role of Estrogen in the Treatment of Men with Schizophrenia‘ provides some more detail:
“The development of the new estrogen compounds – Selective Estrogen Receptor Modulators (SERMs) which do not cause feminisation – opens up the possibility of using a different type of estrogen for a longer period of time at higher doses. Estrogen could therefore prove to be an important component in the treatment of psychotic symptoms in men with schizophrenia. This review explains the scientific rationale behind the estrogen hypothesis and how it can be clinically utilised to address concerns unique to the care of men with schizophrenia.”
Estrogen modulates the release of several monoamines, including serotonin, norepinephrine, dopamine and glutamate, and also enhances acetylcholine levels and inhibits gamma-aminobutyric acid (GABA) receptors. Its effect on these neurotransmitter systems has been likened to that of atypical antipsychotics, and may contribute to the lower illness severity and better outcomes in female patients. [review]
Estrogens are becoming well known for their robust enhancement on cognition particularly for learning and memory that relies upon functioning of the hippocampus and related neural systems. What is also emerging is that estrogen modulation of cognition is not uniform, at times enhancing yet at other times impairing learning. This review explores the bidirectional effects of estrogens on learning from a multiple memory systems view, focusing on the hippocampus and striatum, whereby modulation by estrogens sorts according to task attributes and neural systems engaged during cognition. We highlight our findings that show the ability to solve hippocampus-sensitive tasks typically improves under relatively high estrogen status while the ability to solve striatum-sensitive tasks degrades with estrogen exposures. Though constrained by dose and timing of exposure, these opposing enhancements and impairments of cognition can be observed following treatments with different estrogenic compounds including the hormone estradiol, the isoflavone genistein found in soybeans, and agonists that are selective for specific estrogen receptors, suggesting that activation of a single receptor type is sufficient to produce the observed shifts in learning strategies. Using this multi-dimensional framework will allow us to extend our thinking of the relationship between estrogens and cognition to other brain regions and cognitive functions.
“The hippocampus and striatum are two structures shown to interact competitively. Lesions or chemical inactivation of one system improves learning and memory in tasks that tap the intact structure (Chang and Gold, 2003a and White and McDonald, 2002). Furthermore, the corollary is also true: potentiation of one system may interfere with function of the noncanonical system, impairing cognitive performance that depends on that system. What follows, then, is the possibility that the facilitation of place learning and impairment of response learning by estrogens reflect the singular action of estrogens on either the hippocampus or the striatum that, through competition, leads to the opposing actions. For example, estrogens may improve hippocampal function, thereby impairing striatal function because of competitive inhibition of hippocampus on striatum. Conversely, estrogens may impair striatal function, thereby improving hippocampal function by releasing competitive inhibition of the striatum on hippocampus. Alternatively, estrogens may act on each structure independently, improving place learning through modulation of hippocampus and impairing response learning through modulation of the striatum, without modulating the interactions between memory systems”.
“Estradiol acts through multiple types of estrogen receptors (ER) in the brain. Throughout the brain, ER subtypes ERα and ERβ are detected as classical nuclear receptors but also can be localized to the plasma membrane (Milner et al., 2001, Milner et al., 2005, Shughrue et al., 1997, McEwen et al., 2012, Meitzen and Mermelstein, 2011 and Mhyre and Dorsa, 2006). The G-protein coupled estrogen receptor (GPER), formerly identified as an orphan receptor called GPR30, is a novel seven-transmembrane domain G protein-coupled receptor that binds estradiol with high affinity but is structurally and genetically unrelated to ERα and ERβ (Prossnitz and Barton, 2009). GPER can be located in the plasma membrane and densely expressed in other membrane-bound cellular structures, particularly the endoplasmic reticulum (Brailoiu et al., 2007, Matsuda et al., 2008 and Prossnitz and Barton, 2009). ERα, ERβ, and GPER are all expressed in the hippocampus and striatum, but with distinct nuclear and extra-nuclear distributions and densities, activation through which will lead to different modes of signaling and downstream effects on neural function.
In the hippocampus, classical ERα and ERβ and GPER are detected in relatively high abundance in both nuclear and extranuclear membrane sites in neurons and glia (Brailoiu et al., 2007, Milner et al., 2001, Milner et al., 2005 and Shughrue et al., 1997), allowing for both genomic-initiated transcriptional events and nongenomic- or membrane-initiated signaling pathways. Like estradiol, the highly-specific GPER agonist G1 (Bologa et al., 2006) activates ERK in neurons, increases calcium currents through L-type voltage gated calcium channels (Sun et al., 2010), and increases excitatory post-synaptic currents in hippocampal slices (Lebesgue et al., 2010). In the striatum, while levels of nuclear ERs are strikingly low (Shughrue, 1997), moderate to high levels of ERα, ERβ, and GPER are found at extranuclear sites (Almey et al., 2012, Grove-Strawser et al., 2010, Kuppers and Beyer, 1999 and Schultz et al., 2009). Therefore, it is likely that the primary mode of signaling in the striatum is through membrane-associated ERs or transactivation of other receptors.
Because the mnemonic effects of estrogens are mediated through local actions in the hippocampus or striatum (Zurkovsky et al., 2006, Zurkovsky et al., 2007 and Kent et al., 2005), the differential expression of ER subtypes across these structures may lead to different outcomes on learning when a specific receptor is targeted. Investigating the contributions of distinct ER subtypes to shifts in learning and memory is valuable because certain natural compounds and pharmaceuticals, such as phytoestrogens and selective estrogen receptor modulators (e.g. tamoxifen) bind to estrogen receptors with varying selectivity, and their cognitive effects are largely unknown. Acute treatments of the phytoestrogen genistein mimic the bidirectional effects of estradiol on place and response learning (Pisani et al., 2012b). Genistein demonstrates twenty-fold selectivity for ERβ over ERα (Kuiper et al., 1997), pointing to a possible role of ERβ in both hippocampus-sensitive and striatum-sensitive cognition.
Previous studies implicate the actions of ERβ as critical for the memory enhancing effects of estrogens on radial arm maze (Liu et al., 2008), object recognition (Jacome et al., 2010 and Frick et al., 2010) and social transmission of food preference (Clipperton et al., 2008), but others point to signaling through ERα, especially when hormone treatments were given over short periods (Frye et al., 2007 and Phan et al., 2011), or to activation of both receptors (Hammond et al., 2009 and Qu et al., 2013). A small group of reports shows that activation of GPER can also enhance memory for tasks that engage the hippocampus (Ervin et al., 2013, Gabor et al., 2011, Hammond et al., 2009 and Hawley et al., 2014).
Information regarding the roles of ERα, ERβ, or GPER in hippocampus- or prefrontal cortex-sensitive cognition is accumulating while relatively less is known about the effects of these receptor subtypes on striatum-sensitive learning. The distribution of ERα in extranuclear sites of cholinergic neurons in the striatum and the ability of striatal cholinergic neurons to regulate GABAergic signaling (Almey et al., 2012 and Schultz et al., 2009) suggests that activation of ERα may mediate striatum-sensitive response learning impairments. Hormone loss with ovariectomy increases AMPA receptor density in the striatum (Cyr et al., 2001) that is attenuated by estradiol and ERα agonists but not ERβ agonists (Le Saux et al., 2006). The decrease in AMPA binding may reduce excitatory cortical glutamate input (Davis et al., 2005), decrease striatal synaptic plasticity, and obstruct striatum-sensitive information processing. Sensitive detection methods reveal expression of ERβ predominantly in dopamine afferents to the striatum even though ERα density is substantially higher in the striatum per se ( Almey et al., 2012, Mitra et al., 2003, Creutz and Kritzer, 2002, Shughrue and Merchenthaler, 2001 and Kuppers and Beyer, 1999). ERβ agonists may therefore produce impairments in response learning with expected dose–response functions shifted to the right compared to those for ERα agonists.”
Endogenous 17β-estradiol is required for activity-dependent long-term potentiation in the striatum and interacts with the dopaminergic system [1]. The SERMs, raloxifene and tamoxifen, can prevent dopamine D1/D2 receptor-mediated disruptions of sensorimotor gating, similar to estradiol [2]. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning [3].
Estrogens and mGluRs:
“The rapid effects of estrogens have been shown to be sensitive to G protein manipulation, which suggests that mERα and mERβ may be able to alter G protein receptor activity (Kelly and Wagner 1999). Research suggests that binding at mERα and mERβ stimulates metabotropic glutamate receptors (mGluRs; Meitzen and Mermelstein 2011). mGluRs are G-protein-coupled receptors categorized into three families: mGluRI (mGluR1 and 5) that are Gq receptors, and mGluRII (mGluR2 and 3) and III (mGluR4, 6, and 7), which are Gi/o receptors (Niswender and Conn 2010). In cultured hippocampal and striatal cells, application of E2 increases CREB phosphorylation within 30 seconds, an effect that is mediated by ERα (Boulware et al 2005, Grove-Strawser et al 2010). In cultured hippocampal neurons this effect of E2 estradiol (E2) is replicated by applying an mGluR1 agonist, and blocked by applying an mGluR1 antagonist to the cells (Boulware et al 2005). In contrast, in striatal neurons the E2-induced increase in CREB phosphorylation is mimicked by mGluR5 agonists and blocked by mGluR5 antagonists (Grove-Strawser et al 2010). Additionally E2 can have bidirectional effects on CREB phosphorylation in these brain regions. In hippocampal cultures E2 also inhibits CREB phosphorylation via binding at ERα and ERβ, an effect blocked by mGluR2 antagonists and mimicked by mGluR2 agonists (Boulware et al 2005). In striatal cultures the E2-induced decrease in CREB function is mediated by mGluR3 receptors (Grove-Strawser et al 2010). More recently it was shown that binding at ERα in the CA1 of the hippocampus activates mGluR1, mobilizing components of the endocannabinoid system, leading to reduced GABA release (Tabatadze et al 2013). The authors interpreted these findings to suggest that some of the rapid effects of E2 in the hippocampus and STR are mediated by mER interactions with different members of the mGluRI and mGluRII receptor families (Meitzen and Mermelstein 2011).”
Estrogens alter dopamine-dependent cognitive processes in rats
“There is evidence that estrogens affect many dopamine-dependent cognitive processes, including selective attention object recognition memory and memory system bias. The majority of research has examined the genomic effects of estrogens, administering E2 ~ 12 hours prior to testing, but research examining the rapid effects of E2 on cognition will be described when available. Latent inhibition, a measure of selective attention, is dependent on dopamine transmission within the mesocorticolimbic pathway. Lesions and local infusion of a dopamine antagonist in the PFC enhance latent inhibition (Broersen et al 1996, George et al 2010) whereas lesions of the NAc or hippocampus abolish latent inhibition (Gal et al 1997, Kaye and Pearce,1987, Oswald et al, 2002). Moreover, dopaminergic activity in the anterior STR is positively correlated with behaviour in a latent inhibition task (Jeanblanc et al 2003). Interestingly, increases in plasma levels of estrogens, either during the proestrus phase of the estrous cycle or following E2 replacement in ovariectomized rats, disrupt the expression of latent inhibition (Nofrey et al 2008, Quinlan et al 2010, Almey et al 2013).
Object recognition memory is another dopamine-dependent cognitive process affected by estrogens. Systemic and intra-PFC administration of a D1 antagonist can impair object recognition memory, reflected in poor performance on a novel object preference task (Besheer et al 1999, Nagai et al 2007). Correspondingly, systemic administration of a D1 agonist enhances long-term object recognition memory when administered immediately after training (de Lima et al 2011) or 10 minutes before testing (Hotte et al 2005). Administration of E2 to ovariectomized rats leads to improved object recognition memory (Gervais et al 2013, Jacome et al 2010), an effect that is mimicked by diarylproprionitrile (DPN), an ERβ agonist, suggesting the effects of E2 are at least partially due to binding at ERβ (Inagaki et al 2010, Luine et al 1998). Interestingly, this dose of DPN resulted in a 100% increase in dopamine in the PFC, suggesting that estrogen-induced improvements in recognition memory are due, in part, to increased dopamine (Inagaki et al 2010, Luine et al 1998).
Estrogens also affect the use of place or response memory to navigate an environment. Rats use either spatial or egocentric cues to locate a reward. If spatial cues are used to locate a reward, this is referred to as a place memory, which is mediated by the hippocampus; if egocentric cues are used, this is called a response memory, which is mediated by the dorsal STR (Packard et al 1989, Packard and White 1991, White and McDonald 2002, see also review by Korol and Pisani in this issue). An infusion of amphetamine, an indirect-dopamine agonist, into the hippocampus biases rats toward using place memory, but an amphetamine infusion into the STR biases rats toward using response memory (Packard and White 1991). Interestingly, when estrogen levels are high, either during the proestrus phase of the estrous cycle or following E2 replacement in ovariectomized females, rats are biased toward using place memory (Korol and Kolo 2002, Quinlan et al 2008). More recently we showed that an infusion of E2 directly into the PFC biases female rats towards use of place memory, indicating that E2 acts rapidly (< 15 minutes) to affect memory system bias, possibly via reciprocal projections with the STR and hippocampus (Almey et al 2014). These preclinical studies provide evidence that estrogens affect dopamine-dependent cognitive processes. Some of these cognitive effects of E2 occur rapidly (< 4 hours; Almey et al 2014, Gervais et al 2013, Inagaki et al 2010, Jacome et al 2010), suggesting that the cognitive effects of estrogens result from binding at both nuclear and mERs, leading to genomic and non-genomic effects.”
Estrogens modulate central dopamine transmission
“There is accumulating evidence that estrogens alter dopamine function at various stages in transmission by affecting dopamine availability, dopamine receptor density, and the affinity of the dopamine transporter. Again, the majority of research has examined the genomic effects of estrogens, administering E2 ~ 12 hours prior to testing, but research examining the rapid effects of E2 on dopamine transmission will be described when available. In the STR, tonic dopamine availability is increased when estrogen levels are high (Xiao and Becker 1994); this increase in dopamine is mediated via both long-term and rapid effect of estrogens, as maximal dopamine increases are observed when E2 is administered ~ 12 hours prior to testing, and then again 30 minutes prior to testing (Becker and Rudick 1999). When E2 is applied to tissue from the STR this rapidly decreases dopamine uptake by decreasing the affinity of the dopamine transporter (Disshon et al 1998), providing a potential mechanism for E2-induced increases in dopamine availability. Additionally, ovariectomized rats receiving estrogen replacement have higher binding at dopamine D1 and D2 receptors, but lower binding at D3 receptors, in the STR (Landry et al 2002, Le Saux et al 2006, Levesque and Di Paolo 1989, Levesque et al 1989). This increase in dopamine D2 receptor binding occurs without any changes in dopamine D2 mRNA levels (Le Saux et al 2006), indicating that this change occurs through non-genomic mechanisms.
The relationship between estrogens and dopamine transmission in the NAc is similar to that observed in the STR (Thompson and Moss 1994), as E2 replacement administered to ovariectomized rats is associated with increased tonic (Madularu et al 2014) and phasic (Thompson and Moss 1994) levels of dopamine in the NAc. Systemic administration of E2 increases NAc phasic dopamine release within 15 minutes, indicating this is a rapid effect mediated by mERs (Thompson and Moss 1994). E2 replacement administered to ovariectomized rats was shown to attenuate dopamine reuptake in the NAc (Thompson 1999), providing a potential explanation for the E2-induced dopamine availability in the NAc. Additionally E2 treatment increases dopamine D2 receptor binding in the NAc (Le Saux et al 2006), paralleling findings in the STR.
Estrogens also alter dopamine transmission in the PFC. Females exhibit the highest baseline dopamine levels during estrus, when estrogens are declining, and the lowest basal dopamine levels during proestrus, when estrogen levels are high (Dazzi et al 2007). However, rats in proestrus have higher ethanol-induced dopamine release in the PFC, suggesting that estrogens decrease basal dopamine, but increase dopamine release in the PFC (Dazzi et al 2007). Additionally, dopamine D1 receptor density as well as dendritic spine density is increased in the PFC when ovariectomized females are treated with E2 (Levesque et al 1989; Wallace et al 2006).
The hippocampus receives a small dopaminergic afferent, and more substantial GABAergic afferents from the VTA (Rocchetti et al 2015). Additionally, glutamatergic afferents from the hippocampus to the NAc regulate the firing of dopaminergic neurons in the VTA (Floresco et al 2001). Moreover, hippocampal projections to the PFC are thought to play a role in amplifying neuronal activity in the PFC (Ishikawa and Nakamura 2003). These findings suggest that hippocampal afferents can modulate dopamine activity throughout the mesocorticolimbic pathway.
As discussed extensively in previous reviews (McEwen and Alves, 1999; McEwen and Milner, 2007; Spencer et al., 2008; McEwen et al., 2012), the hippocampus is also sensitive to the effects of estrogen. Female rats, either in the proestrus phase of the estrous cycle or following E2 replacement after ovariectomy had significantly elevated spine synapse density in CA1 pyramidal neurons (Gould et al 1990; Woolley et al 1990). The effect of E2 on synaptic spine density in the hippocampus has been shown to occur rapidly, within 30 minutes of subcutaneous E2 administration (MacLusky et al 2005). In vivo studies demonstrate that female rats administered an infusion of E2 in the hippocampus demonstrate better recollection for the platform location in a watermaze task (Packard and Teather 1997), and demonstrate a bias towards use of place memory to navigate a maze (Zurkovsky et al 2007).”
“Although estrogens have been shown to affect dopamine-dependent cognitive processes in the STR, PFC and hippocampus, these brain are not recognized for having high levels of ERs. …there are rapid behavioural effects of estrogen in the STR, NAc, PFC and hippocampus that could not occur through binding nuclear ERs… binding at GPER1 could be responsible for some of the rapid effects of E2 in these brain regions, but these rapid effects of E2 also suggest mERα or mERβ may be present as well. …The effects of estrogen on cognition likely result from a combination of genomic and non-genomic actions; there is an impetus to clarify the distribution of mERs, to better understand this non-genomic component of estrogen’s effects.”
Estrogens and cholinergic systems
“Studies support the hypothesis that estradiol helps to maintain aspects of attention and verbal and visual memory. Such cognitive domains are exactly those modulated by cholinergic systems and extensive basic and preclinical work over the past several decades has clearly shown that basal forebrain cholinergic systems are dependent on estradiol support for adequate functioning.” [4]
Adjunct treatment with phytoestrogens has been reported to improve symptoms for a patient with schizoaffective disorder [5] and improve depressive symptoms in a case of schizophrenia [6]. The effect of genistein, a phytoestrogen, in a ketamine model of schizophrenia has been studied [7].
For a neurodevelopmental review see: Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain