THE MICROBIOME-THE MISSING LINK IN THE PATHOGENESIS OF SCHIZOPHRENIA
Recent studies indicate that individuals with schizophrenia have evidence of immune activation that may contribute to disease pathogenesis. The source of this immune activation has not been identified but is likely to be related to both genetic and environmental components. Recently it has become apparent that the composition of microbes on mucosal surfaces, termed the microbiome, represents an important modulator of the immune response in humans and in experimental animals. The microbiome has been linked to the generation of an aberrant immune response and alsobeen shown to modulate brain development and behavior in animal model systems. We employed high throughput sequencing to characterize the complete oro-pharyngeal microbiome of 41 individuals with schizophrenia and 32 controls without a psychiatric disorder. We also examined the role of probiotics in modulating the microbiome.Interim analysis indicates that there are large differences between case and control individuals in terms of bacterial, viral, and fungal composition. Individuals with schizophrenia had increased levels of lactic acid bacteria including Lactobacillus casei, Lactobacillus salivarias, Lactobacillus lactis, and Streptococcus thermophilius as well as several other species of streptococci including S mitis and S mutans. Several of these bacteria have been associated with altered Th2 immune responses, an immunological change also noted in schizophrenia. On the other hand individuals with schizophrenia had decreased levels of many non-pathogenic bacteria such as strains of Neisseria, Haemophilus, Prochlorococcus, and Shwanella. Within the group of individuals with schizophrenia, altered levels of microorganisms were associated with an increased prevalence of the deficit syndrome as well as increased levels of intestinal immune activation as indicated by antibodies to food and intestinal antigens. In terms of fungi, individuals with schizophrenia had higher levels of pathogenic yeasts such as Candida glabrata and Candida tropicalis, but lower levels of the relatively less pathogenic Candida albicans. We also characterized a number of known human viruses such as Herpesviruses and Papillomaviruses, as well as bacteriophages and novel viruses. The microbiome was significantly altered by probiotic therapy, with a tendency towards normalization following treatment. Furthermore, many of the species which are increased in the oral microbiome of individuals with schizophrenia, such as streptococci, are modifiable by the administration of antibiotic medications. These studies indicate that the oral microbiome is altered in individuals with schizophrenia and that the microbiome is a potential target for novel therapies.

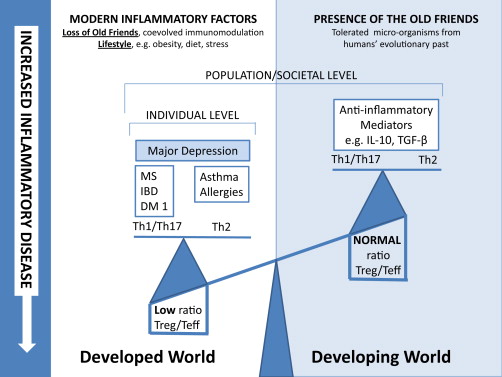
The term ‘‘dysbiosis’’ refers to situations where microbial composition and functions are shifted from their normal beneficial state to another that is deleterious to the host’s health. The microbiota dysbiosis may negatively impact CNS functioning through various intertwined pathways that collectively form the ‘‘brain-gut axis’’. These pathways can be described as follows:
- modification of intestinal permeability that allows entry of endotoxins in the systemic blood flow. The lipopolysaccharide (LPS) is a potent pro-inflammatory endotoxin of the cell walls of gram-negative bacteria that can alter neuronal activity in the limbic system (e.g. increased amygdala activity) and also activate microglia, thus potentially contributing to chronic inflammation in the host’s CNS. Leakage of LPS from the intestine might be a trigger for peripheral inflammatory responses that lead to de novo production of cytokines in the brain. Improving the epithelial barrier may reduce traffic of bacteria and their byproducts and hence be a way to stop the inflammatory response;
- neuropeptides synthesis;
- modulation of local and peripheral inflammation. The gut microbiota regulates the development of lymphoid structures and modulates the differentiation of immune cell subsets thus maintaining homeostatic interactions between the host and the gut microbiota. Certain specific bacteria, including members of the Enterobacteriaceae family, appear to be better equipped for survival under the prevailing conditions in the inflamed gut than are the anaerobic commensals dominant in healthy individuals. Given the postulated anti-inflammatory effects of butyrate, it is possible that depletion in butyrate producing bacteria in dysbiosis may contribute to inflammation. Major depressive disorder, bipolar disorder and schizophrenia are associated with a dysregulation of immune responses as reflected by the observed abnormal profiles of circulating pro- and anti-inflammatory cytokines in affected subjects;
- decrease in absorption of beneficial and essential nutrients (e.g. essential amino acids, vitamins, polyunsaturated fatty acids), increase of deleterious compound synthesis (ammonia, phenols, indoles, sulphide and amines), reduction of the antioxidant status and increase in lipid peroxidation, increase of carbohydrate malabsorption;
- activation/deactivation of the autonomic nervous system that is directly connected to the nucleus tractus solitarius. This nucleus in turn issues direct noradrenergic ascending projections to brain areas involved in anxiety regulation (namely amygdala, basal forebrain cholinergic system and cortex);
- modulation of brain-derived neurotrophic factor;
- increase of small intestinal bacterial overgrowth and/or gastric/instestinal pathogens (e.g. Helicobacter pylori)
Schizophrenia and bipolar disorders
Severance et al. recently measured serological surrogate markers of bacterial translocation (soluble CD14 (sCD14) and lipopolysaccharide binding protein (LBP)) in bipolar subjects (n = 38) and schizophrenia subjects (n = 141) compared to controls. sCD14 seropositivity conferred a 3.1-fold increased odds of association with schizophrenia (OR = 3.09, P < 0.0001) compared to controls. Case–control differences in sCD14 were not matched by LBP. Quantitative levels of LBP, but not sCD14, correlated with BMI in schizophrenia (r2 = 0.21, P < 0.0001). sCD14 and LBP also exhibited some congruency in schizophrenia with both significantly correlated with CRP (P < 0.0001). Antipsychotic treatment generally did not impact sCD14 or LBP levels except for significant correlations, especially sCD14, with gluten antibodies in antipsychotic-naıve schizophrenia (r2 = 0.27, P < 0.0001). In bipolar disorder, sCD14 levels were significantly correlated with anti-tissue transglutaminase IgG (r2 = 0.37, P < 0.001). The authors concluded that these bacterial translocation markers produced discordant patterns of activity that may reflect an imbalanced, activated innate immune state. Whereas both markers may upregulate following systemic exposure to Gram-negative bacteria, autoimmunity, non-lipopolysaccharide-based monocyte activation and metabolic dysfunction may also contribute to the observed marker profiles.
Conclusion
Research on the role of the human intestinal microbiota in the genesis and/or maintenance of psychiatric disorders is in its infancy but appears as one of the most promising avenues of research in psychiatry. While rodent models suggest that the microbiota plays a fundamental role in the genesis of the HPA axis, the serotoninergic system and the immuno-inflammatory system, and that the microbiota can affect the CNS through multiple pathways, few studies have been carried out on humans. Today, autism is the psychiatric disorder in which the role of the microbiota has been the most studied. Some therapeutic opportunities targeting potential microbiota dysbiosis have already been explored such as probiotic administration or diet modifications, with inconsistent results. Further studies are warranted to determine which patients may benefit from microbiota-oriented therapies.
Microbiota Regulation of the Mammalian Gut–Brain Axis
“The findings from clinical studies demonstrate an upregulated immune and inflammatory status in patients with schizophrenia (Song et al., 2013) and a correlation between the level of inflammatory markers and severity of clinical symptoms (Hope et al., 2013). It has been suggested that the uncontrolled neuroinflammation by proinflammatory cytokines is involved in the pathogenesis of schizophrenia (Dennison et al., 2012 and Nemani et al., 2014). Chronic macrophage activation and secretion of interleukin-2 and interleukin-2 receptors has been proposed as the basic biological mechanism of schizophrenia in earlier papers (Smith, 1991 and Smith, 1992). For example, the protozoa Toxoplasma gondii is known to cause major perturbation to the gut microbiota and is a recognized environmental risk factor for schizophrenia ( Bhadra et al., 2013 and Molloy et al., 2013). More recently a chlorovirus (family Phycodnaviridae) has been identified in humans that affects cognitive function relevant to schizophrenia in animal models ( Yolken et al., 2014).
NMDA receptor hypofunction is believed to be central to the pathophysiology of schizophrenia, as NMDA receptor antagonists produce schizophrenia-like symptoms while agents that enhance NMDA receptor function reduce negative symptoms and improve cognition (Coyle, 2012). Variation in BDNF expression is believed to play a role in the molecular mechanism underlying cognitive dysfunction in schizophrenia (Nieto, Kukuljan, & Silva, 2013). Given that normal development of the microbiota is necessary to stimulate brain plasticity through the appropriate expression of BDNF and NMDA receptors, it is possible that altered microbiota may contribute to the NMDA receptor dysfunction seen in schizophrenia (Dinan et al., 2014 and Nemani et al., 2015). In animal model of schizophrenia (chronic NMDA antagonist treatment), it has been shown that the gut microbiota profile correlated to memory performance, suggesting an influence of the microbiota on cognition in the model, which was supported by restoration of cognition through oral ampicillin administration (Pyndt Jorgensen et al., 2014).
Evidence showing possible microbiota alteration in schizophrenia includes structural damage to the GI tract, a heightened immune response to infectious pathogens and food antigens, and known differences in the microbiome in other neuropsychiatric disorders (Nemani et al., 2014). Further investigation into the microbiota and how the gut–brain axis may mediate the link between neuropsychiatric disease and the immune system is needed.
It is also worth noting that one of the most important side effects of treatments for schizophrenia is weight gain and metabolic syndrome. We have recently shown that the microbiota plays a critical role in olanzapine-induced weight gain in rats (Davey et al., 2013 and Davey et al., 2012) which has been confirmed in germ-free mice study (Morgan et al., 2014)”
“Considering the gut’s multifaceted capacity to communicate with the CNS, it is plausible that the gut and its components are playing a crucial role in resultant mood and behaviors. Some therapeutic opportunities targeting potential microbiota dysbiosis have already been explored such as probiotic administration, fecal transplantation, or diet modifications, with inconsistent results. Exciting evidence from animal studies has provided the rationale to warrant further exploration in humans, both in health and disease. Future research should focus on delineating the relative contributions of immune, neural, and endocrine pathways through which the gut microbiota communicates with the brain. A better understanding of these pathways will improve our knowledge about the role of gut microbiota play in a range of neurological disorders, including neuropsychiatric diseases”
Schizophrenia and the gut–brain axis
Potential novel therapeutics for schizophrenia
“The composition of the microbiome in schizophrenia has yet to be investigated, but there are several therapeutic interventions on the horizon which have less potential for toxicity than traditional treatment and may exert their influence through altering the microbiota.
Dietary interventions
Dietary changes affect both the composition and function of the gut microbial communities, which in turn can alter the innate and adaptive immune system (Vieira et al., 2014). Seven clinical trials have been published that examine the effects of a gluten-free diet on schizophrenia symptoms (Kalaydjian et al., 2006). These early studies included schizophrenia patients not tested for antibodies and had variable results. The resolution of schizophrenia symptoms after initiation of a gluten-free diet has been described in case reports (De Santis et al., 1997, Jackson et al., 2012, Jansson et al., 1984 and Kraft and Westman, 2009), and there are at least two current trials underway that are investigating the effects of gluten removal on schizophrenia symptoms in AGA-positive individuals. Data from these trials and future studies are needed to determine the impact of gluten-free diet on the subpopulation of people with schizophrenia who are sensitive to gluten.
While a study conducted in 1973 showed earlier discharge from the hospital in relapsed schizophrenia patients after a cereal-free, milk-free diet (Dohan and Grasberger, 1973), the impact of a casein-free diet in isolation on schizophrenia has yet to be investigated.
Antimicrobials
Alteration of the gut commensal microbiota with antibiotics has been shown to modify the susceptibility to autoimmune demyelinating processes of the central nervous system, such as that seen in multiple sclerosis (Ochoa-Repáraz et al., 2009). The protection afforded antimicrobials is associated with a shift in the immune response from Th1/Th17 toward anti-inflammatory Th2-type responses (Ochoa-Repáraz et al., 2010). Minocycline, a second generation tetracycline, is currently under investigation as an adjunct treatment in schizophrenia and there has been some early evidence of its efficacy in treating negative symptoms (Jhamnani et al., 2013, Khodaie-Ardakani et al., 2014 and Liu et al., 2014) and treatment-resistant schizophrenia (Qurashi et al., 2014). While tetracycline is believed to have a neuroprotective effect due to its anti-inflammatory action and ability to enhance glutamate neurotransmission (Liu et al., 2014), its immunomodulatory properties as mediated by gut microbiota have yet to be investigated and may play a role.
Probiotics
There is promising clinical evidence to support a role of probiotic interventions in reducing anxiety, decreasing the stress response, and improving mood in both animals (Arseneault-Bréard et al., 2012, Bravo et al., 2011 and Messaoudi et al., 2011) and humans (Logan and Katzman, 2005, Messaoudi et al., 2011 and Rao et al., 2009). The mechanism responsible for these effects is not known, but it has been hypothesized that it may be related to a reduction in the effects of pro-inflammatory cytokines as well as modification in nutritional status through direct effects on B vitamins, omega-3 fatty acids, and minerals (Cryan and O’Mahony, 2011 and Logan and Katzman, 2005). Probiotics have also been found to improve lactose digestion and potentially reduce the interference that high intestinal lactose concentration may have with serotonin availability through l-tryptophan metabolism (Ledochowski et al., 1998). As patients with schizophrenia often suffer from an increased stress response, compromised nutritional status, increased inflammatory status, and lactose sensitivity, probiotic interventions have promising therapeutic potential.
Probiotic interventions have also been shown to improve obesity-associated dyslipidemia and insulin resistance in animal models (Yu et al., 2013) and reduce weight gain and fat mass (Ji et al., 2012). Oral administration of Lactobacillus gasseri SBT2055 in healthy overweight humans has been found to reduce abdominal visceral and subcutaneous fat ( Kadooka et al., 2010). The anti-obesity effects of probiotics may have therapeutic potential for patients with schizophrenia given their higher risk metabolic profile. A recent study in rats reported that co-administration of antibiotics attenuated olanzapine-induced alteration in microbiota, as well as olanzapine-induced metabolic disturbances including weight gain, visceral fat deposition, elevated plasma free fatty acids, and macrophage infiltration of adipose tissue ( Davey et al., 2013). These findings suggest that microbiota might be a novel treatment target for metabolic comorbidity in patients with schizophrenia.
Fecal microbiota transplantation
While probiotics and antibiotics may be the best known and commercially available options to treat gastrointestinal dysbiosis, fecal microbiota transplantation is an old procedure that has been rediscovered as a cutting-edge option for the restoration of gut microbiota. There has been growing interest in the use of fecal microbiota for the treatment of patients with chronic gastrointestinal infections and inflammatory bowel diseases. Lately, there has also been interest in its therapeutic potential for extraintestinal conditions including cardiometabolic disease and autoimmune disorders (Smits et al., 2013). A better understanding of the microbiome in schizophrenia needs to be gained before the therapeutic potential of fecal transplantation can be explored.”
Evidence that patients with schizophrenia may have altered microbiota:
1. Changes in the microbiota seen in neuropsychiatric disorders
2. Structural damage to the GI tract in schizophrenia
3. Abnormal response to infectious pathogens in schizophrenia
4. Abnormal response to food antigens in schizophrenia
5. Sensitivity to gluten and bovine casein“Several risk factors for the development of schizophrenia can be linked through a common pathway in the intestinal tract. A growing body of research emphasizes the role of the microbiota in regulating brain development, immune function, and metabolism but the composition of the microbiome in individuals with schizophrenia has yet to be investigated. Evidence that indicates possible microbiota alteration in schizophrenia includes structural damage to the GI tract, a heightened immune response to infectious pathogens and food antigens, and known differences in the microbiome in other neuropsychiatric disorders. A significant subgroup of patients may benefit from the initiation of a gluten and casein-free diet, and the therapeutic potential of antimicrobial and probiotic interventions will be elucidated by further research. Further investigation into the microbiome and how the gut may mediate the link between neuropsychiatric disease and the immune system is needed.”
Although peripheral immune system abnormalities have been linked to schizophrenia pathophysiology, standard antipsychotic drugs show limited immunological effects. Thus, more effective treatment approaches are required. Probiotics are microorganisms that modulate the immune response of the host and, therefore, may be beneficial to schizophrenia patients. The aim of this study was to examine the possible immunomodulatory effects of probiotic supplementation in chronic schizophrenia patients. The concentrations of 47 immune-related serum proteins were measured using multiplexed immunoassays in samples collected from patients before and after 14 weeks of adjuvant treatment with probiotics (Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12; n = 31) or placebo (n = 27). Probiotic add-on treatment significantly reduced levels of von Willebrand factor (vWF) and increased levels of monocyte chemotactic protein-1 (MCP-1), brain-derived neurotrophic factor (BDNF), RANTES, and macrophage inflammatory protein-1 beta (MIP-1) beta with borderline significance (P ≤ 0.08). In silico pathway analysis revealed that probiotic-induced alterations are related to regulation of immune and intestinal epithelial cells through the IL-17 family of cytokines. We hypothesize that supplementation of probiotics to schizophrenia patients may improve control of gastrointestinal leakage.
The gut microbiome has been shown to regulate the development and functions of the enteric and central nervous systems. Its involvement in the regulation of behavior has attracted particular attention because of its potential translational importance in clinical disorders, however little is known about the pathways involved. We previously have demonstrated that administration of Lactobacillus rhamnosus (JB-1) to healthy male BALB/c mice, promotes consistent changes in GABA-A and -B receptor sub-types in specific brain regions, accompanied by reductions in anxiety and depression-related behaviors. In the present study, using magnetic resonance spectroscopy (MRS), we quantitatively assessed two clinically validated biomarkers of brain activity and function, glutamate+glutamine (Glx) and total N-acetyl aspartate+N-acetylaspartylglutamic acid (tNAA), as well as GABA, the chief brain inhibitory neurotransmitter. Mice received 10(9) JB-1 per day for 28days and were subjected to MRS weekly and again 4weeks after cessation of treatment to ascertain temporal changes in these neurometabolites. Baseline concentrations for Glx, tNAA and GABA were equal to 10.4±0.3mM, 8.7±0.1mM, and 1.2±0.1mM, respectively. Delayed increases were first seen for Glx (~10%) and NAA (~37%) at 2weeks which persisted only to the end of treatment. However, Glx was still elevated 4weeks after treatment had ceased. Significantly elevated GABA (~25%) was only seen at 4weeks. These results suggest specific metabolic pathways in our pursuit of mechanisms of action of psychoactive bacteria. They also offer through application of standard clinical neurodiagnostic techniques, translational opportunities to assess biomarkers accompanying behavioral changes induced by alterations in the gut microbiome.
Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice.
Ingestion of specific probiotics, namely “psychobiotics”, produces psychotropic effects on behavior and affects the hypothalamic-pituitary-adrenal axis and neurochemicals in the brain. We examined the psychotropic effects of a potential psychobiotic bacterium, Lactobacillus plantarum strain PS128 (PS128), on mice subjected to early life stress (ELS) and on naïve adult mice. Behavioral tests revealed that chronic ingestion of PS128 increased the locomotor activities in both ELS and naïve adult mice in the open field test. In the elevated plus maze, PS128 significantly reduced the anxiety-like behaviors in naïve adult mice but not in the ELS mice; whereas the depression-like behaviors were reduced in ELS mice but not in naïve mice in forced swimming test and sucrose preference test. PS128 administration also reduced ELS-induced elevation of serum corticosterone under both basal and stressed states but had no effect on naïve mice. In addition, PS128 reduced inflammatory cytokine levels and increased anti-inflammatory cytokine level in the serum of ELS mice. Furthermore, the dopamine level in the prefrontal cortex (PFC) was significantly increased in PS128 treated ELS and naïve adult mice whereas serotonin (5-HT) level was increased only in the naïve adult mice. These results suggest that chronic ingestion of PS128 could ameliorate anxiety- and depression-like behaviors and modulate neurochemicals related to affective disorders. Thus PS128 shows psychotropic properties and has great potential for improving stress-related symptoms
Bidirectional brain gut microbial interactions
A recent animal study may shed some light on pathological bidirectional ‘brain-gut-axis’ interactions in schizophrenia:
Subchronic phencyclidine (subPCP) treatment induces schizophrenic-like behavior in rodents, including cognitive deficits and increased locomotor sensitivity towards acute administration of PCP. Evidence is accumulating that the gut microbiota (GM) influences behavior through modulation of the microbiota–gut–brain axis, and hence, part of the variation within this animal model may derive from variation in the GM. The aims of this study was to investigate first, the duration of subPCP-induced cognitive impairment in the novel object recognition test, and second, the possible effect of subchronic PCP-treatment on the GM, and the association between the GM and the behavioral parameters. The association was further investigated by antibiotic reduction of the GM. Male Lister Hooded rats were dosed twice daily i.p. with either 5 mg/kg PCP or sterile isotonic saline for seven days followed by a seven-day washout period. Rats were tested in the novel object recognition and the locomotor activity assays immediately after, three weeks after, or six weeks after washout, and the fecal GM was analyzed by high throughput sequencing. Antibiotic- and control-treated rats were tested in the same manner following washout. In conclusion, subPCP-treatment impaired novel object recognition up to three weeks after washout, whereas locomotor sensitivity was increased for at least six weeks after washout. Differences in the core gut microbiome immediately after washout suggested subPCP treatment to alter the GM. GM profiles correlated to memory performance. Administration of ampicillin abolished the subPCP-induced memory deficit. It thus seems reasonable to speculate that the GM influences memory performance, contributing to variation within the model.
“The core gut microbiome of subPCP rats differed significantly from that of vehicle-treated rats immediately after washout, suggesting a PCP-induced stress-modulation of the GM. However, this cannot be fully concluded based on the setup of the study, where rats were co-housed. Single housing was not preferred, as single housing previously has been shown to impact rodent memory performance. Thus, the core gut microbiome was compared in an attempt to overcome a possible effect of the cage-factor on the GM. By this approach only bacteria which were present in at least 50% of the samples were included in the analysis, thereby eliminating the effect of bacteria only being present in a single cage. No difference was seen between treatment groups when assessed at three weeks after washout, suggesting stabilization within three weeks after washout. The GM of SubPCP rats tended to contain increased abundance of the genera Roseburia, Dorea, and Odoribacter immediately after washout compared to vehicle-treated rats. Roseburia spp. and Dorea spp. have previously been shown to be affected by stress induced by social defeat in mice, where time between the stress factor and fecal sampling seemed to be an important factor affecting the abundance of especially Roseburia spp. Moreover, we have also reported an increase in Odoribacter as a consequence of stress exposure in mice. The present observations are in line with the previous ones, thus, supporting the hypothesis that subPCP administration induces stress-related changes in the GM. However, the relevance and the influence of these bacteria on behavior are not clear, and consequently, further investigations into this should be conducted to clarify the relevance of the observations. Three weeks after washout the GM of SubPCP rats tended to contain increased abundance of an unclassified genus from the S24-7 family compared to vehicle-treated rats. A previous study found increased abundance of an unclassified genus of this family to be associated with improved spatial memory performance. This is in contradiction to the present finding, where SubPCP rats display decreased memory performance. However, it can be speculated whether the tendency towards an increase in abundance of this genus reflects the initial phase of a rise in abundance, contributing in restoring memory formation, and so, studies addressing this would be interesting to perform.
The GM profiles correlated significantly to memory performance immediately after washout, and showed a tendency towards the same three weeks after washout, suggesting an additive influence from the GM on the behavior observed in the model. Based on this, it is proposed that variation within the GM contributes to the variation observed in memory performance within the model. However, additional studies addressing this association need to be performed for further conclusion. Therefore, the second study was set up, in which the GM was substantially reduced by oral ampicillin-administration throughout the whole study period, in order to investigate whether a modulation of the GM would affect behavior in the model.
Administration of ampicillin to SubPCP rats restored the memory performance, but did not affect locomotor activity. This observation supports the correlation between the GM and memory formation found in the first study, suggesting an association between the GM and memory performance in the model, in which the commensal GM seems to be important for induction of the PCP-induced deficits. Some β-lactam antibiotics, to which ampicillin belongs, have been demonstrated to be able to increase expression of the glutamate transporter GLT-1 protein in the central synapses. Upregulation of GLT-1 reduces synaptic glutamate, thus contributing to decreased activation of the NMDA receptor, which enhances the effect of the NMDA receptor antagonist PCP. The effect of this does not seem to be of importance in the present study, in which the negative effect of PCP on cognition was abolished in ampicillin-treated rats, whereas the locomotor activity was not affected in either treatment groups.
Mimicking the NMDA receptor hypofunction hypothesis, administration of the NMDA receptor antagonist PCP affects the inhibitory GABA interneuron signaling, which is thought to be involved in the pathogenesis of schizophrenia. Reduced expression of hippocampal parvalbumin containing GABA neurons has been demonstrated six weeks after subPCP treatment. Habitants of the gut are able to affect levels of central neurotransmitters and receptors, as reviewed by Collins. As an example, administration of the commensal Lactobacillus rhamnosus has been shown to influence central GABA receptor expression in mice, which coincided with reduced stress-induced corticosterone levels and relief of depression- and anxiety-like behavior, where vagotomy inhibited the effect on brain and behavior. It can be speculated that altering the GM through antibiotic treatment in the present study may influence the level of specific GABA receptors, secondly impacting behavior. Another factor involved in brain plasticity and cognition is brain-derived neurotrophic factor (BDNF). Several studies demonstrate the ability of the GM to modulate central levels of BDNF. It can be speculated whether this neurotrophic factor is involved in restoration of the subPCP-induced brain neurochemical changes. Thus, it can be suggested that an ampicillin-induced alteration of the GM may affect cognition of SubPCP rats through modulation of BDNF levels in the present study. M. Gareau et al. demonstrated reduced levels of hippocampal BDNF and impaired memory in the NOR test of germ-free mice when compared to SPF mice, which illustrates the impact of the presence of the GM on brain development and memory formation. Demonstrating the impact of the composition of the GM, memory deficits in the NOR were abolished by probiotic treatment of stress exposed SPF mice. Furthermore, Bercik et al. found oral antibiotic treatment of SPF mice to decrease anxiety behavior and increase central BDNF levels. Neither intraperitoneal antibiotic treatment of SPF mice nor oral antibiotic treatment of germ-free mice had an effect on behavior, thus, demonstrating the impact of the GM on behavior and the brain. Vagotomy or chemical sympathectomy did not affect the impact of the GM, and neither was local intestinal inflammation found to be involved, and consequently, they proposed GM metabolites to be involved in the communication between the gut and the brain. Unfortunately, the effect of intraperitoneal antibiotic administration on central BDNF levels was not addressed. The studies support the results of the present study demonstrating oral antibiotic modulation of the GM to relieve subPCP-induced cognitive deficits, suggesting influence from the gut commensals to be through modulation of brain receptors, neurotransmitters, and BDNF. Hence, it seems plausible to speculate that the GM affects behavior in the subchronic PCP-induced animal model of schizophrenia. However, future studies should clarify whether an unknown, direct central effect of ampicillin is involved in restoring cognition before final conclusions on the present observations can be drawn.”
“GM analysis suggested the gut microbiome to be affected by subchronic PCP-treatment immediately after washout, with restoration within three weeks. The GM profile correlated to memory performance in the NOR, suggesting an influence of the GM on cognition in the model, which was supported by restoration of cognition in SubPCP rats through oral ampicillin administration. Further studies are needed to conclude more on this. In perspective, if the GM impacts memory performance, as the study suggests, this knowledge may be used to refine the animal model.”
See also:
Dietary glycemic index as a modulator of behavioral and biochemical abnormalities?
For more information:
Host microbiota constantly control maturation and function of microglia in the CNS
Serotonin, tryptophan metabolism and the brain-gut-microbiome axis
Diet-Microbe Interactions in the Gut
Collective unconscious: How gut microbes shape human behavior.
Adult Hippocampal Neurogenesis Is Regulated by the Microbiome
Commentary: Gut Microbiota and Brain Function: A New Target for Brain Diseases?

Dear Madam/Sir,
I want to ask permission to use your very informative illustration (with reference) of the bi-directional brain-gut microbial interactions in an article on irritable bowel syndrome for the medical journal Gut Microbes.
Best regards
Hans Raskov, M.D.
Copenhagen
Denmark
LikeLike
Hi – you’d best ask the authors of that particular article (contact emayer@ucla.edu). Any reference should be made to http://dx.doi.org/10.1053/j.gastro.2014.02.037
LikeLike